we are going to cover Food Chemistry Multiple Choice Questions | Food Question and Answer or food and drink Quiz. Introduction and quiz is given below.
Food Chemistry
The study of the chemicals that go into making a product is the focus of the science discipline of chemistry. Food chemistry is the study of the chemicals present in food, how they affect human nutrition, and how they can be modified or mixed to produce new foods. Most likely, you have seen the nutritional information on a food packaging. The substances that make up the meal you are consuming are mentioned under the ingredients.
Food Labelling
Any tag, brand, mark, visual representation, or other descriptive information that is written, stencilled, printed, embossed, marked, or impressed on, or connected to, a container of food or food product is regarded as a food label in accordance with the accepted definition.
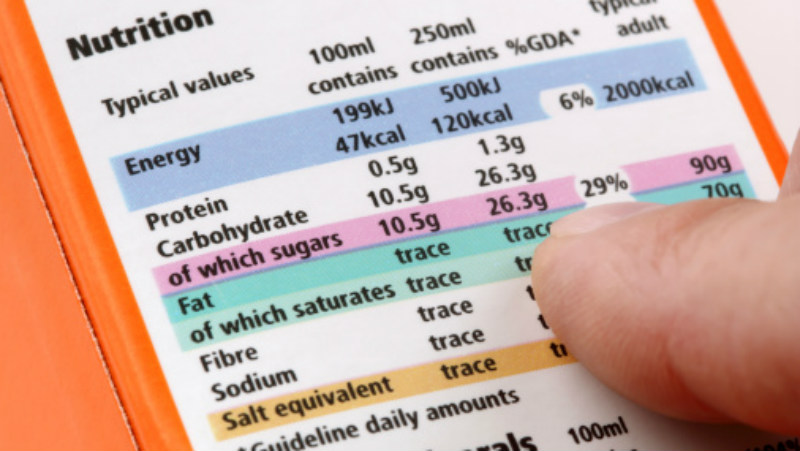
Ingredient list on food label
Ingredient list on food label including Serving Size, Cholesterol, Total Calories, Sodium, Carbohydrates – Sugar and Fiber, Fats – Trans and Saturated, Vitamins, Protein, and Other Nutrients.
Yellow 6
Sudan I is sulfonated in Yellow 6, a hazardous chemical. One of the first petroleum azo compound dyes created in the late 19th century during the European textile boom was this deadly chemical.
fd&c Yellow 5
Since many years ago, tartrazine, also known as fd&c Yellow 5, has been a widely used artificial food colour in both food and medicine.
Purple food
Blackberries, Forbidden rice, Eggplant, Purple sweet potatoes, Purple cauliflower, Redbor kale, Elderberries, Purple carrots, Acai berries, Purple mangosteen, Passion fruit, Purple barley, Purple asparagus, Purple star apple, Red dragon fruit, etc., are including in Purple food category.
fd&c dyes
Innumerable combinations of FD&C (Food, Drugs and Cosmetics) dyes and lakes, sometimes referred to as certified or artificial colours, can be used to create almost any hue possible for cosmetic items.
fd&c yellow 6
A synthetic colour made from petroleum called fd&c Yellow 6 has FDA approval for use in food, medicine, and cosmetics.
White Food
White Bread, White Pasta, White Rice, Yogurt, White Sugar, Salt, White Potatoes, Animal-Based Fats, Turnips, Onions, Mushrooms, Garlic, Cauliflower, White Beans, Parsnips, White Fish, Coconut, Milk, Cheese, Egg Whites, etc., are including in white food items.
Purple Carrot Cake
A healthier alternative of traditional carrot cake is purple carrot cake. Similar to orange carrot cake in terms of moistness and flavour, but with less sugar and oil, as well as a surprising number of health benefits that can help you beat the winter blues or purples.
Making purple with food coloring
For making purple with food coloring add 80 drops of red food colouring and fifteen drops of blue food colouring.
Red Food
Tomato, Apples, Red Bell Pepper, Strawberries, Watermelon, Red Kidney Beans, Cherries, Red Cabbage, Beet Root, Pomegranates, Raspberries, Red Onions, Cranberries, etc., are including in red foods.
Red Coloring
A synthetic food dye or colourant derived from petroleum is called red coloring. The Food and Drug Administration (FDA) has approved nine certified colour additives for use in foods and beverages. Additionally, it is permitted for use as a food colourant in the European Union.
Artificial Food Coloring
The majority of foods that are coloured artificially use dyes, which are petroleum-based synthetic substances that are not found in nature are known as artificial food coloring. A reasonable rule of thumb is to avoid all meals that have been coloured because food dyes are almost exclusively employed in foods with little nutritional content (candy, gelatin desserts, soft drinks, etc.).
Caramel e150d
By heating a carbohydrate with food-grade sulfite and ammonium chemicals, caramel e150d is produced. From light brown to dark black-browns, the colour is varied. The strongest negative charge is displayed by class IV caramels over a wide pH range, giving them the most adaptable caramel tints.
Annatto Colour
The achiote tree, which grows in tropical areas of South and Central America, produces the seeds that are used to make the orange-red food colouring or condiment known as annatto color.
Food Question and Answer
1. Which protein fraction of egg is having the antibiotic property?
a. Lysozyme
b. Ovomucoid
c. Ovomucin
d. Avidin
Ans. a
2. If buffers are present, the rate of browning reaction:
a. Decreases
b. Increases
c. Remains Constant
d. Can not be pridicted
Ans. b
3. As per EU’s CN code maltodextrin have DE value:
a. Less than 5
b. Less than 20
c. Less than 50
d. More than 50
Ans. b
4. Agar is:
a. Polypeptide
b. Polysaccharide
c. Polyphenol
d. Polyflavone
Ans. b
5. The temperature at which the solution remains in the equilibrium with crystalline solvent and the crystalline solute is:
a. Transition temperature
b. Crystalline temperature
c. Eutectic temperature
d. Melting temperature
Ans. c
Food Chemistry Important Questions | Easy Food Quiz Questions and Answers
6. A substance incorporated into a polymeric material to increase its deformality is called:
a. Stablizer
b. Emulsifier
c. Destabilzer
d. Plastiizer
Ans. d
7. Which of the following statements is/are correct?
a. A true solvent is always a plasticizer
b. A plasticizer is always a true solvent
c. A plasticizer increases the glass transition temperature of a polymer
d. All of the above
Ans. a
8. Subtle changes in the structure, which do not drastically alter the molecular architecture of protein, are usually regarded as:
a. Conformational adaptability
b. Denaturation
c. Putrefaction
d. Emulsification
Ans. a
9. Which of the following statements is/are correcct?
a. Partially denatured proteins are more digestible
b. Partially denatured proteins have better emulsifying property
c. Partially denatured proteins have better foaming properties
d. All of the above
Ans. d
10. What is the meaning of “two-state transition” of globular proteins?
a. Globular proteins can exist only in the native and denatured states
b. Globular proteins at their isoelectric point transit from globular to fibrous from
c. Globular proteins can be present in two forms-solid and liquid at transition temperature
d. None of the above
Ans. a
Food & Drink quiz questions
11. Which of the following statements is/are correct?
a. Proteins of the thermophilic organisms usually contains large amount of hydrophobic amino acids residues
b. Thermal denaturation of monomeric globular proteins is mostly reversible
c. Dry proteins powders are extremely stable to thermal denaturation
d. All of the above
Ans. d
12. The protein portion of the myoglobin is called:
a. Heme
b. Globin
c. Flavone
d. Myoglobin does not contain any protein
Ans. b
13. How many myoglobin molecules are linked together in a hemoglobin molecule?
a. Two
b. Three
c. Four
d. Five
Ans. c
14. Increase in the number of -OH group attached to the anthocyanin:
a. Increase its reddish colour
b. Decrease its reddish colour
c. Increase its blue colour
d. Increase both reddish and bluish colour
Ans. c
15. Anthocyanins are stable at:
a. Neutral pH
b. Acidic pH
c. Basi pH
d. Independent of pH
Ans. b
16. In sorption isotherm, the boundary of Zones 1 and 2 corresponds to the:
a. BET monolayer
b. PET monolayer
c. CET monolayer
d. MET monolayer
Ans. a
17. Which of the following is/are the mode of non-enzymic browning in food?
a. Caramelization
b. Maillard reaction
c. Ascorbic acid oxidation
d. All of the above
Ans. d
18. Which of the following is/are the functions of anthocyanins?
a. Antioxidant
b. Colorant
c. Anticancer
d. All of the above
Ans. d
19. Which of the following statements is/are correct with respect to the pigments?
a. Flavonoids are water soluble pigment
b. Basic structure of carotenoids is isoprene
c. Blair process is also to retain green color of the chlorophyll during processing
d. All of the above
Ans. d
General knowledge food quiz
20. Colorant used in butter is:
a. Annato
b. Erythrosine
c. Congo red
d. None of the above
Ans. a
21. The chemically annato is:
a. Carotenoids
b. Flavonoids
c. Heme pigments
d. None of the above
Ans. a
22. Bixin is used as colorant in:
a. Chocolate
b. Cola beverages
c. Butter
d. All of the above
Ans. c
23. Which of the following statements is/are correct?
a. Anthocyanin contains sugar molecule
b. Anthocyanidin contains sugar molecule
c. Glycosylation of anthocyanin increases the wavelength of the absorption spectra
d. All of the above
Ans. a
24. A fruit discoloration problem referred to as “pinking” is the result of:
a. Microbial spoilage of the product
b. Formation of metal anthocyanin complex
c. Biochemical changes in the fruits
d. None of the above
Ans. b
25. Amadori rearrangement is found during:
a. Protein biosynthesis
b. Fat p oxidation
c. Maillard reaction
d. Kreb cycle
Ans. c
Chemical Food Preservatives | Food Additives and Preservatives
26. “Pinking” can be avoided by:
a. Blanching
b. AR enamel
c. Treatment of fruits with vinegar
d. Blairs process
Ans. b
27. The compound formed at elevated pH by the reaction between ammonia and magnesium in case of pea is:
a. Struvite
b. Magnetite
c. Appetite
d. None of the these
Ans. a
28. Thermal degradation of glutamine leads to the formation of:
a. Pyrrolidone citric acid
b. Pyrrolidone carboxylic acid
c. Pyrrolidone acetic acid
d. None of the these
Ans. b
29. Increase in acidity of vegetables during the thermal processing is due to:
a. Formation of pyrollidone carboxylic acid
b. Degradation of chlorophyll
c. Decrease in natural butter
d. All of the above
Ans. a
30. Which of the following relationship regarding the rate of thermal destruction is correct?
a. Chlorophyll a< chlorophyll b< Clostridium botulinum
b. Chlorophyll b< chlorophyll a< Clostridium botulinum
c. Clostridium botulinum< chlorophyll b< chlorophyll a
d. None of the above
Ans. b
Citric acid preservative | Organic chemical used in agricultural of food chemistry
31. Compound formed by the action of chlorophyllase on chlorophyll is:
a. Pheophytin
b. Pheophorbide
c. Chlorophyllide
d. Mesochlorophyll
Ans. c
32. Which of the following statements is correct?
a. Mesochlorophyll and chlorophyll differ only in terms of CH3 group
b. Mesochlorophyll and chlorophyll differ in terms of CHO group
c. Mesochlorophyll and chlorophyll differ in terms of Mg ion
d. None of the above
Ans. a
33. After the removal of Mg ion from chlorophyll, the compound formed is:
a. Pheophytin
b. Pheophorbide
c. Chlorophillide
d. Mesochlorophyll
Ans. a
34. If both Mg ion and phyton chain are removed from chlorophyll, the compound formed is:
a. Cheophytin
b. Pheophorbide
c. Chlorophillide
d. Pyrophorbide
Ans. b
35. Chlorophillide differs from chlorophyll on the basis that fromer lack:
a. Mg ion
b. Phytol chain
c. Both a and b
d. None of the above
Ans. b
36. At low pH, chlorophyll degrades to:
a. Pheophytin
b. Carboxylic acid
c. Pyropheophytin
d. Mesochlorophyll
Ans. a
37. Color of the compound formed after the removal of Mg ion from chlorophyll is:
a. Green
b. Olive green
c. Brown
d. Black
Ans. b
38. Schiff base in the intermediate formed during:
a. Caramelization
b. Ascorbic acid oxidation
c. Enzymic browning
d. Maillard reaction
Ans. d
39. Commercially available copper complex of pheophorbide, used as food colorant is:
a. Copper chlorophyll
b. Copper chlorophillide
c. Copper chlorophyllin
d. None of the above
Ans. c
40. “Ragreening” of thermal processed vegetables is:
a. Formation of the bright green color in presence of Zn and Cu
b. Loss of green color
c. Gaining green color after its loss
d. None of the above
Ans. a
41. The metal ion used in Veri-green process is:
a. Sodium
b. Magnesium
c. Zinc
d. Calcium
Ans. c
42. Green color in vegetables processed in the presence of zinc is largely due to the presence of:
a. Zinc chlorophyllin
b. Zinc chlorophillide
c. Zinc pheophorbide
d. Zinc pyropheophytin
Ans. d
43. Which of the following carotenoids show/s pro-vitamin A activity?
a. P-carotene
b. a-carotene
c. P-crptoxanthin
d. All of the above
Ans. a
44. Who explained the structure of protein?
a. Emil Fischer
b. Pauling and Corey
c. R.F. Rose
d. Johnson and Cristae
Ans. b
food and drink quiz questions
45. Primary structure of proteins is/are:
a. Open chain
b. Cyclic
c. Branched chain
d. All of the above
Ans. d
46. In cyclic primary structure of protein:
a. There is no terminal -COOH group
b. There is one terminal -COOH group
c. There are two terminal -COOH groups
d. Any of the above
Ans. a
47. Which of the following proteins has/have cyclic primary structure?
a. Tyrocidine
b. Ubiquitin
c. Keratin
d. All of the above
Ans. a
48. Which of the following words is related sorption isotherm during rehydration of a dry food?
a. Graining
b. Nucleation
c. Hysteresis
d. Solvation
Ans. c
49. What is the unit of water activity?
a. Gram
b. Gram/litre
c. It is unit less
d. None of the above
Ans. c
50. The peptide bond has:
a. Plant structure
b. Angular structure
c. Tetrahedral structure
d. Pyramidal structure
Ans. a
chemistry of preservatives | food preservatives examples
51. Most of the unsaturated fatty acids arein cis-geometrical configuration. But there are certain exceptions like:
a. Lactobacillic acid
b. Tuberculostearic acid
c. Crebronic acid
d. All of the above
Ans. d
52. Which of the following equation is used to explain enzyme kinetics?
a. Reynold’s equation
b. Micchaelis-Menten equation
c. Arrhenius equation
d. Boyle’s equation
Ans. b
53. Trans form of oleic acid is:
a. Linoleic accid
b. Linolenic acid
c. Elaidic acid
d. Oleic acid is in itself trans form
Ans. c
54. Oleic acid can be transferred into its trans form upon:
a. Cooling
b. Heating
c. Solidification
d. None of the above
Ans. b
55. Triglycerides, which are solid at room temperature are often referred as:
a. Fat
b. Oil
c. Steroids
d. Waxes
Ans. a
56. Triglycerides, which are liquid at room temperature are often referred as:
a. Fat
b. Oil
c. Steroids
d. Waxes
Ans. b
Ascorbic acid preservative
57. In hard water, whicch of the following salta is/are present?
a. Sodium chloride
b. Magnesium sulphate
c. Sodium biarbonate
d. All of the above
Ans. b
58. Castor seed is rich in:
a. Ricinoleic acid
b. Oleic acid
c. Linolenic acid
d. Linoleic acid
Ans. a
59. Glucosyl acylglycerol contains sugar. This sugar molecule is bound to glycerol by:
a. Peptide linkage
b. Glycosidicc bond
cc. Hydrogen bond
d. Disulphide bond
Ans. b
60. The parent compound in phospho glycerides is:
a. Phosphoric acid
b. Phosphoric ester of glycerol
c. Phosphoric ester of Fatty acid
d. Glycerol
Ans. b
61. The nitrogen containing compound in cephalin is:
a. Guanine
b. Ethanolamine
c. Choline
d. Ammonium acetate
Ans. b
62. Ovomucoid is the protein found in:
a. Fruits
b. Vegetables
c.Milk
d. Egg
Ans. d
63. The nitrogen containing compound in leithin is:
a. Guanine
b. Ethanolamine
c. Choline
d. Ammonium acetate
Ans. c
64. In enzyme kinetics, Michaelis constant is:
a. the substrate concentration at which velocity of reaction is maximum
b. the substrate concentration at which velocity of reation is half of maximum velocity
c. the substrate concentration at which velocity of reaction is one-fourth of maximum velocity
d. None of the above
Ans. b
65. Hydrolysis of triglycerides with alkali is called:
a. Titration
b. Hydrogenation
c. Saponification
d. Rancidity
Ans. c
66. Major component of beewax is:
a. Palmitic acid
b. Oleic acid
c. Stearic acid
d. Linoleni acid
Ans. a
67. Ovomucoid is found to be:
a. Antibiotic in nature
b. Trypsin inhibitor
c. Hemaglutination inhibitor
d. Iron binder
Ans. b
68. Which of the following is wool fat:
a. Shingosine
b.Sialon
c. Lanolin
d. Ceramides
Ans. c
69. A plot of water content of a food versus relative vapour pressure at constant temperature is known as:
a. Moisture absorption plot
b. Moisture sorption isotherm
c. Moisture pressure slope
d. Water activity plot
Ans. b
70. Which fatty acids are more odorous?
a. Low molecular weight fatty acids
b. Medium molecular weight fatt acids
c. High molecular weight fatty acids
d. All are equally odorous
Ans. a
71. Iodine value measures:
a. Degree of unsaturation
b. Degree of saturation
c. Amount of carbon present
d. Number of Iodine present
Ans. a
72. What is dilatometry?
a. Measurement of degree of unsaturation of fatty acids
b. Measurement of degree of hydrogenation
c. Measurement of melting points of fats
d. Measurement of crystallinity of fats
Ans. d
73. Which of the following gives the value of free fatty acids present in fat?
a. Iodine value
b. Acid value
c. Saponification value
d. Peroxide value
Ans. b
74. Which of the following is said to be haemagglutination inhibitor?
a. Lysozyme
b. Ovomucin
c. Avidin
d. Conalbumin
Ans. b
75. With the increase in temperature, the rate of browning reaction:
a. Increases
b. Decrease
c. Remains constant
d. First decreases and then increases followed by a constant phase
Ans. a
76. Peroxide value is the measure of:
a. Degree of unsaturation
b. Degree of saturation
c. Amount of carbon present
d. Degree of oxidation
Ans. d
77. The average molecular weight of fatty acid is determined by:
a. Iodine value
b. Acid value
c. Saponification value
d. Peroxide value
Ans. c
78. Hydrolytic rancidity of fat requires:
a. Oxygen
b. Moisture
c. High temperature
d. Both b and c
Ans. d
79. Final product of rancidity is:
a. Oxides
b. Peroxides
c. Hydroperoxides
d. Carbon dioxide
Ans. c
80. Which vitamin is absent in egg?
a. Vitamin A
b. Vitamin C
c. Vitamin D
d. Vitamin K
Ans. b
81. Rancidity is progressed through the formation of:
a. Free radicals
b. Carbocations
c. Carboanions
d. Carbenes
Ans. a
82. For hydrogenation of oils, the catalyst required is:
a. Iron
b. Aluminium
c. Nickel
d. Magnesium
Ans. c
83. Hydrogenation of oils is carried out in a vessel called:
a. Hydrogenator
b. Converter
c. Reverter
d. Condenser
Ans. b
84. Hydrogenation is addition of hydrogen to ……………… in presence of some catalyst:
a. Saturated fatty acids
b. Unsaturated fatty aids
c. Both a and b
d. None
Ans. b
85. Trans fatty acids are found in some plant oils such as:
a. Pomegranate oil
b. Mustard oil
c. Coconut oil
d. Citrus oil
Ans. a
86. Conalbumin is said to bind with:
a. Iron
b. Calcium
c. Cobalt
d. Avidin
Ans. a
87. The development of hydrogenation process is credited to French chemist:
a. Appart
b. Lohmann
c. Sabatier
d. Oldman
Ans. c
88. Which of the following sugars do you expect to have higher affinity for non-enzymic browning?
a. Aldohexoses
b. Aldopentoses
c. Disaccharides
d. Non-reducing sugars
Ans. a
89. Which of the following statements is/are correct?
a. Old or poisoned catalysts produce finished product with higher levels of trans fatty acids
b. Trans fatty acids are having low melting point than their respective cis form
c. Hydrogenation formis cis form of the fatty acids
d. All of the above
Ans. a
Food Chemistry Question Bank
90. Among the toxic fatty acid have received the greatest attention:
a. Elaidic acid
b. Erucic acid
c. Myristic acid
d. Lignoceric acid
Ans. b
91. Roquefortine is:
a. Bacterial toxin
b. Mycotoxin
c. Antinutritional factor
d. A fermented product
Ans. b
92. Which of the following is the biotin binder?
a. Avidin
b. Aflatoxin
c. Gossypol
d. Ovalbumin
Ans. a
93. Roqueofortine is found in:
a. Cheese
b. Meat
c. Egg
d. Fruits
Ans. a
94. Which of the following pigments is responsible for the yellow color in corn?
a. Xanthophyll
b. Chlorophyll
c. Zeaxanthin
d. Cryptoxanthan
Ans. c
Multiple Choice Questions on Food Chemistry
95. Gelatin is:
a. Product obtained from agar-agar
b. Partially degraded protein
c. Important constituent of jelly responsible for its characteristic gel structure
d. None of the above
Ans. b
96. Gelatin is obtained from:
a. Bones, skin and ligaments
b. Egg shell
c. Agar-agar
d. Papaya
Ans. a
97. When collodial dispersion of some relatively large molecules are cooled, the viscosity increases to a point at which some rigidity is attained. This point is called:
a. Gel point
b. Viscous point
c. Coagulation point
d. None of the above
Ans. b
98. Rocky candy aroma is obtained when glucose reacts with:
a. Aspartic acid
b. Lauric acid
c. Sulphuric acid
d. Citric acid
Ans. a
99. Most abundant mineral present in the egg shell is:
a. Iron
b. Magnesium
c. Zinc
d. Calcium
Ans. d
100. Snake venom phopholipase is used for the determination of:
a. Degree of unsaturation of fatty acids
b. Positional distribution of fatty acids in acylglycerol molecules
c. Stability of triglycerides
d. All of the above
Ans. b


:max_bytes(150000):strip_icc()/food-and-chemistry-101635580-577bf82a5f9b585875b46d64.jpg)