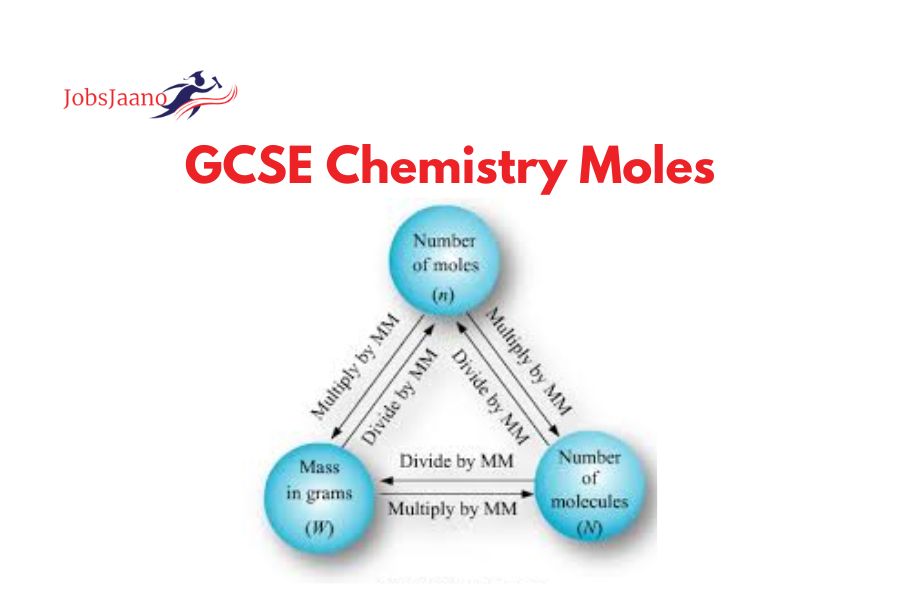
GCSE Chemistry Moles Questions and Answers pdf: we will discuss GCSE Chemistry’s Moles topic.
GCSE Chemistry Moles Questions and Answers | GCSE chemistry calculations questions and answers pdf
Question 1: How many moles of water (H₂O) molecules are present in 18 grams of water?
Answer: To determine the number of moles, divide the given mass by the molar mass of water.
Molar mass of water (H₂O) = 2(1.008) + 16.00 = 18.016 g/mol
Moles of water = 18 g / 18.016 g/mol ≈ 0.998 moles
Question 2: How many atoms are present in 0.5 moles of sodium (Na)?
Answer: To determine the number of atoms, multiply the number of moles by Avogadro’s number.
Number of atoms of sodium (Na) = 0.5 moles × 6.022 × 10²³ atoms/mol ≈ 3.011 × 10²³ atoms
Question 3: How many moles of carbon dioxide (CO₂) are produced when 1 mole of methane (CH₄) is completely burned?
Answer: The balanced equation for the combustion of methane is:
CH₄ + 2O₂ → CO₂ + 2H₂O
From the equation, it can be seen that 1 mole of methane produces 1 mole of carbon dioxide.
Question 4: What is the molar mass of calcium chloride (CaCl₂)?
Answer: Molar mass of calcium chloride (CaCl₂) = Atomic mass of calcium (Ca) + 2 × Atomic mass of chlorine (Cl)
Atomic mass of calcium (Ca) = 40.08 g/mol
Atomic mass of chlorine (Cl) = 35.45 g/mol
Molar mass of calcium chloride = 40.08 g/mol + 2 × 35.45 g/mol = 110.98 g/mol
Question 5: How many grams of magnesium (Mg) are required to react with 0.5 moles of hydrochloric acid (HCl)?
Answer: The balanced equation for the reaction between magnesium and hydrochloric acid is:
Mg + 2HCl → MgCl₂ + H₂
From the equation, it can be seen that 1 mole of magnesium reacts with 2 moles of hydrochloric acid.
Therefore, 0.5 moles of hydrochloric acid will require 0.25 moles of magnesium.
To find the mass of magnesium, multiply the moles by the molar mass of magnesium.
Question 6: How many moles of oxygen gas (O₂) are required to react completely with 0.25 moles of glucose (C₆H₁₂O₆) in cellular respiration?
Answer: The balanced equation for cellular respiration is:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
From the equation, it can be seen that 1 mole of glucose reacts with 6 moles of oxygen.
Therefore, 0.25 moles of glucose will require 6 × 0.25 = 1.5 moles of oxygen.
Question 7: What is the molar mass of sulfuric acid (H₂SO₄)?
Answer: Molar mass of sulfuric acid (H₂SO₄) = 2 × Atomic mass of hydrogen (H) + Atomic mass of sulfur (S) + 4 × Atomic mass of oxygen (O)
Atomic mass of hydrogen (H) = 1.008 g/mol
Atomic mass of sulfur (S) = 32.06 g/mol
Atomic mass of oxygen (O) = 16.00 g/mol
Molar mass of sulfuric acid = 2 × 1.008 g/mol + 32.06 g/mol + 4 × 16.00 g/mol = 98.09 g/mol
Question 8: How many atoms are present in 0.2 moles of carbon dioxide (CO₂)?
Answer: To determine the number of atoms, multiply the number of moles by Avogadro’s number.
Number of atoms in carbon dioxide (CO₂) = 0.2 moles × 6.022 × 10²³ atoms/mol = 1.204 × 10²³ atoms
Question 9: How many grams of hydrochloric acid (HCl) are required to react with 2.5 moles of sodium hydroxide (NaOH)?
Answer: The balanced equation for the reaction between hydrochloric acid and sodium hydroxide is:
HCl + NaOH → NaCl + H₂O
From the equation, it can be seen that 1 mole of sodium hydroxide reacts with 1 mole of hydrochloric acid.
Therefore, 2.5 moles of sodium hydroxide will require 2.5 moles of hydrochloric acid.
To find the mass of hydrochloric acid, multiply the moles by the molar mass of hydrochloric acid.
Question 10: How many moles of carbon dioxide (CO₂) are produced when 5 moles of ethane (C₂H₆) are combusted completely?
Answer: The balanced equation for the combustion of ethane is:
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
From the equation, it can be seen that 2 moles of ethane produce 4 moles of carbon dioxide.
Therefore, 5 moles of ethane will produce (4/2) × 5 = 10 moles of carbon dioxide.
GCSE chemistry questions and answers pdf | jee mains questions on mole concept
Question 11: How many moles of oxygen gas (O₂) are produced when 0.8 moles of potassium chlorate (KClO₃) decompose?
Answer: The balanced equation for the decomposition of potassium chlorate is:
2KClO₃ → 2KCl + 3O₂
From the equation, it can be seen that 2 moles of potassium chlorate produce 3 moles of oxygen gas.
Therefore, 0.8 moles of potassium chlorate will produce (3/2) × 0.8 = 1.2 moles of oxygen gas.
Question 12: What is the molar mass of carbon dioxide (CO₂)?
Answer: Molar mass of carbon dioxide (CO₂) = Atomic mass of carbon (C) + 2 × Atomic mass of oxygen (O)
Atomic mass of carbon (C) = 12.01 g/mol
Atomic mass of oxygen (O) = 16.00 g/mol
Molar mass of carbon dioxide = 12.01 g/mol + 2 × 16.00 g/mol = 44.01 g/mol
Question 13: How many molecules are present in 2 moles of methane (CH₄)?
Answer: To determine the number of molecules, multiply the number of moles by Avogadro’s number.
Number of molecules of methane (CH₄) = 2 moles × 6.022 × 10²³ molecules/mol = 1.204 × 10²⁴ molecules
Question 14: How many grams of copper (Cu) are needed to produce 0.4 moles of copper(II) oxide (CuO)?
Answer: The balanced equation for the reaction between copper and oxygen to form copper(II) oxide is:
2Cu + O₂ → 2CuO
From the equation, it can be seen that 2 moles of copper react to produce 2 moles of copper(II) oxide.
Therefore, 0.4 moles of copper will produce (2/2) × 0.4 = 0.4 moles of copper(II) oxide.
To find the mass of copper, multiply the moles by the molar mass of copper.
Question 15: How many moles of sulfuric acid (H₂SO₄) are required to neutralize 15 grams of sodium hydroxide (NaOH)?
Answer: The balanced equation for the neutralization reaction between sulfuric acid and sodium hydroxide is:
H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O
From the equation, it can be seen that 1 mole of sulfuric acid reacts with 2 moles of sodium hydroxide.
To find the moles of sulfuric acid, divide the given mass of sodium hydroxide by its molar mass, and then multiply by the mole ratio.
Question 16: How many moles of carbon atoms are present in 1 mole of ethane (C₂H₆)?
Answer: Since there are two carbon atoms in one mole of ethane, the number of moles of carbon atoms is also 1 mole.
Question 18: How many molecules are present in 2 moles of water (H₂O)?
Answer: To determine the number of molecules, multiply the number of moles by Avogadro’s number.
Number of molecules of water (H₂O) = 2 moles × 6.022 × 10²³ molecules/mol = 1.204 × 10²⁴ molecules.
Question 19: How many grams of oxygen gas (O₂) are needed to react completely with 5 moles of magnesium (Mg)?
Answer: The balanced equation for the reaction between magnesium and oxygen is:
2Mg + O₂ → 2MgO
From the equation, it can be seen that 1 mole of magnesium reacts with 1/2 mole of oxygen.
Therefore, 5 moles of magnesium will require 1/2 × 5 = 2.5 moles of oxygen.
To find the mass of oxygen, multiply the moles by the molar mass of oxygen.
Question 20: How many moles of hydrogen gas (H₂) are produced when 3 moles of zinc (Zn) react with excess hydrochloric acid (HCl)?
Answer: The balanced equation for the reaction between zinc and hydrochloric acid is:
Zn + 2HCl → ZnCl₂ + H₂
From the equation, it can be seen that 1 mole of zinc produces 1 mole of hydrogen gas.
Therefore, 3 moles of zinc will produce 3 moles of hydrogen gas.
Mole concept questions class 11 | NEET questions on mole concept
Question 21: What is the molar mass of carbon monoxide (CO)?
Answer: Molar mass of carbon monoxide (CO) = Atomic mass of carbon (C) + Atomic mass of oxygen (O).
Atomic mass of carbon (C) = 12.01 g/mol
Atomic mass of oxygen (O) = 16.00 g/mol
Molar mass of carbon monoxide = 12.01 g/mol + 16.00 g/mol = 28.01 g/mol.
Question 22: How many atoms are present in 0.25 moles of calcium (Ca)?
Answer: To determine the number of atoms, multiply the number of moles by Avogadro’s number.
Number of atoms of calcium (Ca) = 0.25 moles × 6.022 × 10²³ atoms/mol.
Question 23: How many grams of sodium chloride (NaCl) are produced when 1 mole of sodium (Na) reacts with excess chlorine gas (Cl₂)?
Answer: The balanced equation for the reaction between sodium and chlorine is:
2Na + Cl₂ → 2NaCl
From the equation, it can be seen that 2 moles of sodium produce 2 moles of sodium chloride.
Therefore, 1 mole of sodium will produce 1 mole of sodium chloride.
To find the mass of sodium chloride, multiply the moles by the molar mass of sodium chloride.
Question 24: How many moles of nitrogen gas (N₂) are produced when 10 moles of ammonia (NH₃) decompose?
Answer: The balanced equation for the decomposition of ammonia is:
2NH₃ → N₂ + 3H₂
From the equation, it can be seen that 2 moles of ammonia produce 1 mole of nitrogen gas.
Therefore, 10 moles of ammonia will produce (1/2) × 10 = 5 moles of nitrogen gas.
Question 25: How many molecules are present in 0.5 moles of carbon dioxide (CO₂)?
Answer: To determine the number of molecules, multiply the number of moles by Avogadro’s number.
Number of molecules of carbon dioxide (CO₂) = 0.5 moles × 6.022 × 10²³ molecules/mol.
Question 26: How many moles of oxygen gas (O₂) are required to completely react with 2 moles of propane (C₃H₈) in combustion?
Answer: The balanced equation for the combustion of propane is:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
From the equation, it can be seen that 1 mole of propane requires 5 moles of oxygen gas.
Therefore, 2 moles of propane will require 5 × 2 = 10 moles of oxygen gas.
Question 27: What is the molar mass of calcium carbonate (CaCO₃)?
Answer: Molar mass of calcium carbonate (CaCO₃) = Atomic mass of calcium (Ca) + Atomic mass of carbon (C) + 3 × Atomic mass of oxygen (O)
Atomic mass of calcium (Ca) = 40.08 g/mol
Atomic mass of carbon (C) = 12.01 g/mol
Atomic mass of oxygen (O) = 16.00 g/mol
Molar mass of calcium carbonate = 40.08 g/mol + 12.01 g/mol + 3 × 16.00 g/mol = 100.09 g/mol
Question 28: How many molecules are present in 0.25 moles of methane (CH₄)?
Answer: To determine the number of molecules, multiply the number of moles by Avogadro’s number.
Number of molecules in methane (CH₄) = 0.25 moles × 6.022 × 10²³ molecules/mol
Question 29: How many grams of sodium hydroxide (NaOH) are needed to react with 1 mole of hydrochloric acid (HCl)?
Answer: The balanced equation for the reaction between sodium hydroxide and hydrochloric acid is:
NaOH + HCl → NaCl + H₂O
From the equation, it can be seen that 1 mole of sodium hydroxide reacts with 1 mole of hydrochloric acid.
To find the mass of sodium hydroxide, multiply the moles by the molar mass of sodium hydroxide.
Question 30: How many moles of carbon dioxide (CO₂) are produced when 4 moles of glucose (C₆H₁₂O₆) undergo complete combustion?
Answer: The balanced equation for the combustion of glucose is:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
From the equation, it can be seen that 1 mole of glucose produces 6 moles of carbon dioxide.
Therefore, 4 moles of glucose will produce 6 × 4 = 24 moles of carbon dioxide.
Questions on mole concept class 11 pdf | Mole concept mcq for neet pdf
Question 31: What is the molar mass of nitrogen gas (N₂)?
Answer: Since nitrogen gas exists as a diatomic molecule (N₂), the molar mass is the sum of the atomic masses of two nitrogen atoms.
Atomic mass of nitrogen (N) = 14.01 g/mol
Molar mass of nitrogen gas (N₂) = 2 × 14.01 g/mol = 28.02 g/mol
Question 32: How many atoms are present in 0.5 moles of hydrogen gas (H₂)?
Answer: To determine the number of atoms, multiply the number of moles by Avogadro’s number.
Number of atoms in hydrogen gas (H₂) = 0.5 moles × 6.022 × 10²³ atoms/mol
Question 33: How many grams of sulfuric acid (H₂SO₄) are needed to neutralize 25 grams of sodium hydroxide (NaOH)?
Answer: The balanced equation for the neutralization reaction between sulfuric acid and sodium hydroxide is:
H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O
From the equation, it can be seen that 1 mole of sulfuric acid reacts with 2 moles of sodium hydroxide.
To find the grams of sulfuric acid, multiply the moles by the molar mass of sulfuric acid.
Question 34: How many moles of carbon atoms are present in 1 mole of glucose (C₆H₁₂O₆)?
Answer: Since glucose has 6 carbon atoms in its formula, the number of moles of carbon atoms is also 6 moles.
Question 35: How many molecules are present in 0.5 moles of nitrogen gas (N₂)?
Answer: To determine the number of molecules, multiply the number of moles by Avogadro’s number.
Number of molecules in nitrogen gas (N₂) = 0.5 moles × 6.022 × 10²³ molecules/mol
Question 36: How many moles of water (H₂O) are produced when 2 moles of hydrogen gas (H₂) react with excess oxygen (O₂)?
Answer: The balanced equation for the reaction between hydrogen gas and oxygen to form water is:
2H₂ + O₂ → 2H₂O
From the equation, it can be seen that 2 moles of hydrogen gas produce 2 moles of water.
Therefore, 2 moles of hydrogen gas will produce 2 moles of water.
Question 37: What is the molar mass of sodium sulfate (Na₂SO₄)?
Answer: Molar mass of sodium sulfate (Na₂SO₄) = 2 × Atomic mass of sodium (Na) + Atomic mass of sulfur (S) + 4 × Atomic mass of oxygen (O)
Atomic mass of sodium (Na) = 22.99 g/mol
Atomic mass of sulfur (S) = 32.07 g/mol
Atomic mass of oxygen (O) = 16.00 g/mol
Molar mass of sodium sulfate = 2 × 22.99 g/mol + 32.07 g/mol + 4 × 16.00 g/mol = 142.04 g/mol
Question 38: How many molecules are present in 1 mole of carbon monoxide (CO)?
Answer: To determine the number of molecules, multiply the number of moles by Avogadro’s number.
Number of molecules of carbon monoxide (CO) = 1 mole × 6.022 × 10²³ molecules/mol
Question 39: How many grams of sulfur dioxide (SO₂) are produced when 2 moles of sulfur (S) react with excess oxygen (O₂)?
Answer: The balanced equation for the reaction between sulfur and oxygen to form sulfur dioxide is:
S + O₂ → SO₂
From the equation, it can be seen that 1 mole of sulfur produces 1 mole of sulfur dioxide.
Therefore, 2 moles of sulfur will produce 2 moles of sulfur dioxide.
To find the mass of sulfur dioxide, multiply the moles by the molar mass of sulfur dioxide.
Question 40: How many moles of oxygen gas (O₂) are required to react completely with 1 mole of ethene (C₂H₄) in combustion?
Answer: The balanced equation for the combustion of ethene is:
C₂H₄ + 3O₂ → 2CO₂ + 2H₂O
From the equation, it can be seen that 3 moles of oxygen gas are required to react with 1 mole of ethene.
Therefore, 1 mole of ethene will require 3 moles of oxygen gas.
Mole concept class 11 questions jee | mole concept mcq for neet pdf
Question 41: What is the molar mass of hydrochloric acid (HCl)?
Answer: Molar mass of hydrochloric acid (HCl) = Atomic mass of hydrogen (H) + Atomic mass of chlorine (Cl)
Atomic mass of hydrogen (H) = 1.008 g/mol
Atomic mass of chlorine (Cl) = 35.45 g/mol
Molar mass of hydrochloric acid = 1.008 g/mol + 35.45 g/mol = 36.46 g/mol
Question 42: How many atoms are present in 0.1 moles of carbon dioxide (CO₂)?
Answer: To determine the number of atoms, multiply the number of moles by Avogadro’s number.
Number of atoms in carbon dioxide (CO₂) = 0.1 moles × 6.022 × 10²³ atoms/mol
Question 43: How many grams of potassium nitrate (KNO₃) are needed to produce 3 moles of oxygen gas (O₂) through decomposition?
Answer: The balanced equation for the decomposition of potassium nitrate is:
2KNO₃ → 2KNO₂ + O₂
From the equation, it can be seen that 2 moles of potassium nitrate produce 1 mole of oxygen gas.
Therefore, 3 moles of oxygen gas will require (1/2) × 3 = 1.5 moles of potassium nitrate.
To find the mass of potassium nitrate, multiply the moles by the molar mass of potassium nitrate.
Question 44: How many moles of ammonia (NH₃) are produced when 2 moles of nitrogen gas (N₂) react with excess hydrogen gas (H₂)?
Answer: The balanced equation for the reaction between nitrogen gas and hydrogen gas to form ammonia is:
N₂ + 3H₂ → 2NH₃
From the equation, it can be seen that 1 mole of nitrogen gas produces 2 moles of ammonia.
Therefore, 2 moles of nitrogen gas will produce 2 × 2 = 4 moles of ammonia.
Question 45: How many molecules are present in 0.2 moles of sulfuric acid (H₂SO₄)?
Answer: To determine the number of molecules, multiply the number of moles by Avogadro’s number.
Number of molecules of sulfuric acid (H₂SO₄) = 0.2 moles × 6.022 × 10²³ molecules/mol
Question 46: How many moles of oxygen gas (O₂) are produced when 4 moles of potassium chlorate (KClO₃) decompose?
Answer: The balanced equation for the decomposition of potassium chlorate is:
2KClO₃ → 2KCl + 3O₂
From the equation, it can be seen that 2 moles of potassium chlorate produce 3 moles of oxygen gas.
Therefore, 4 moles of potassium chlorate will produce (3/2) × 4 = 6 moles of oxygen gas.
Question 47: What is the molar mass of hydrofluoric acid (HF)?
Answer: Molar mass of hydrofluoric acid (HF) = Atomic mass of hydrogen (H) + Atomic mass of fluorine (F)
Atomic mass of hydrogen (H) = 1.008 g/mol
Atomic mass of fluorine (F) = 18.998 g/mol
Molar mass of hydrofluoric acid = 1.008 g/mol + 18.998 g/mol = 20.006 g/mol
Question 48: How many molecules are present in 0.5 moles of carbon dioxide (CO₂)?
Answer: To determine the number of molecules, multiply the number of moles by Avogadro’s number.
Number of molecules in carbon dioxide (CO₂) = 0.5 moles × 6.022 × 10²³ molecules/mol
Question 49: How many grams of aluminum (Al) are needed to produce 4 moles of aluminum oxide (Al₂O₃) through oxidation?
Answer: The balanced equation for the oxidation of aluminum to form aluminum oxide is:
4Al + 3O₂ → 2Al₂O₃
From the equation, it can be seen that 4 moles of aluminum produce 2 moles of aluminum oxide.
Therefore, 4 moles of aluminum will require (2/4) × 4 = 2 moles of aluminum oxide.
To find the mass of aluminum, multiply the moles by the molar mass of aluminum.
Question 50: How many moles of chlorine gas (Cl₂) are needed to react with 1 mole of sodium (Na) to form sodium chloride (NaCl)?
Answer: The balanced equation for the reaction between chlorine and sodium to form sodium chloride is:
2Na + Cl₂ → 2NaCl
From the equation, it can be seen that 1 mole of sodium reacts with 1/2 mole of chlorine gas.
Therefore, 1 mole of sodium will require (1/2) × 1 = 0.5 moles of chlorine gas.
Question 51: How many atoms are present in 0.3 moles of methane (CH₄)?
Answer: To determine the number of atoms, multiply the number of moles by Avogadro’s number.
Number of atoms in methane (CH₄) = 0.3 moles × 6.022 × 10²³ atoms/mol
Question 52: How many grams of carbon dioxide (CO₂) are produced when 3 moles of ethane (C₂H₆) are burned completely?
Answer: The balanced equation for the combustion of ethane is:
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
From the equation, it can be seen that 2 moles of ethane produce 4 moles of carbon dioxide.
Therefore, 3 moles of ethane will produce (4/2) × 3 = 6 moles of carbon dioxide.
To find the mass of carbon dioxide, multiply the moles by the molar mass of carbon dioxide.
Question 53: How many moles of hydrogen gas (H₂) are produced when 0.5 moles of magnesium (Mg) react with excess hydrochloric acid (HCl)?
Answer: The balanced equation for the reaction between magnesium and hydrochloric acid is:
Mg + 2HCl → MgCl₂ + H₂
From the equation, it can be seen that 1 mole of magnesium produces 1 mole of hydrogen gas.
Therefore, 0.5 moles of magnesium will produce 0.5 moles of hydrogen gas.
Question 54: How many molecules are present in 0.1 moles of sulfuric acid (H₂SO₄)?
Answer: To determine the number of molecules, multiply the number of moles by Avogadro’s number.
Number of molecules of sulfuric acid (H₂SO₄) = 0.1 moles × 6.022 × 10²³ molecules/mol
Related Queries: