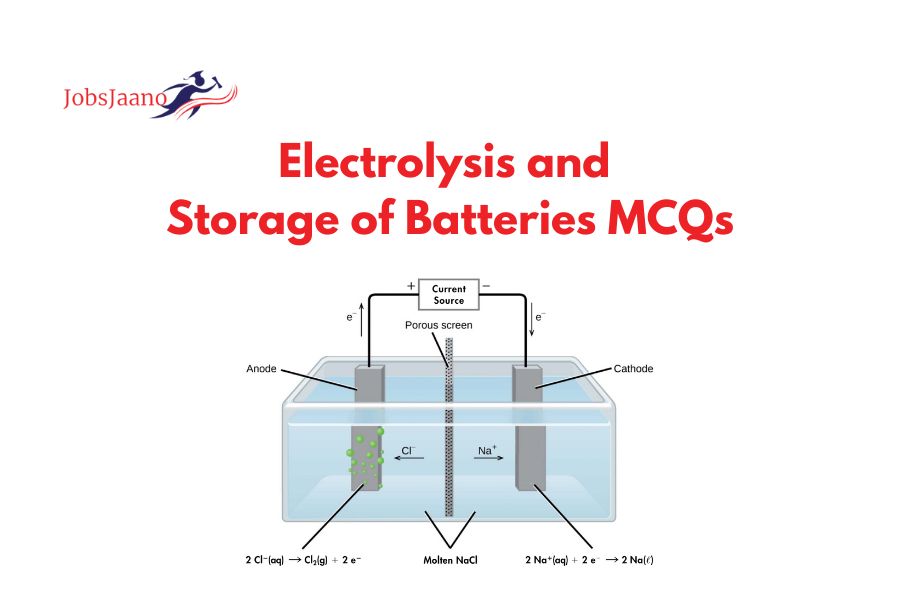
MCQs on Electrolysis and Storage of Batteries (Electricity Engineering): Electrolysis and the storage of batteries are two separate concepts related to energy conversion and storage. Let me explain each of them:
Electrolysis: It is a procedure that utilizes current to drive a non-spontaneous chemical reaction. It is commonly used to split water molecules into hydrogen and oxygen gases. This process occurs in an electrolyzer, which consists of an electrolyte solution (such as water) and two electrodes. When an electric current is passed through the solution, positive ions migrate towards the negative electrode (cathode), and negative ions migrate towards the positive electrode (anode).
At the cathode, hydrogen gas (H2) is produced, while at the anode, oxygen gas (O2) is produced.
2H2O(l) → 2H2(g) + O2(g)
Electrolysis has applications in various fields, such as hydrogen production for fuel cells, chemical synthesis, and energy storage. The produced hydrogen can be stored and later used as a fuel source, either directly or by converting it back into electricity using fuel cells.
Battery Storage: Battery storage involves the use of rechargeable batteries to store electrical energy. They consist of one or more electrochemical cells, which contain positive and negative electrodes immersed in an electrolyte.
During charging, a voltage is applied to the battery, causing a chemical reaction that stores energy by converting it into electrochemical potential energy. This energy can be stored and later released as electrical energy when needed. The most common types of rechargeable batteries include lead-acid batteries, lithium-ion batteries, and nickel-metal hydride batteries.
Battery storage systems are widely used in various applications, such as electric vehicles, portable electronics, renewable energy integration, and backup power supplies. They provide a convenient way to store and release energy, offering flexibility in terms of usage and mobility.
It’s worth noting that while electrolysis and batteries both involve the use of electrochemical reactions, they serve different purposes. Electrolysis is primarily used for the production of hydrogen gas or other chemical compounds, while batteries are focused on the storage and release of electrical energy.
Electrolysis MCQs | Battery MCQ pdf
1. Which of the following processes uses an electric current to split water into hydrogen and oxygen gases?
a) Electroplating
b) Electrolysis
c) Electromagnetism
d) Electrostatics
Answer: b) Electrolysis
2. What is the primary purpose of electrolysis?
a) Production of electrical energy
b) Production of hydrogen gas
c) Charging batteries
d) Extraction of metals from ores
Answer: b) Production of hydrogen gas
3. Which type of battery is commonly used in electric vehicles?
a) Lead-acid battery
b) Nickel-metal hydride battery
c) Lithium-ion battery
d) Alkaline battery
Answer: c) Lithium-ion battery
4. What is the main function of a battery storage system?
a) Conversion of electrical energy into chemical energy
b) Conversion of mechanical energy into electrical energy
c) Storage and release of electrical energy
d) Production of hydrogen gas
Answer: c) Storage and release of electrical energy
5. Which of the following is not an application of battery storage?
a) Electric vehicles
b) Portable electronics
c) Hydrogen production
d) Renewable energy integration
Answer: c) Hydrogen production
6. Which of the following is the most common electrolyte used in water electrolysis?
a) Sodium chloride
b) Sulfuric acid
c) Potassium hydroxide
d) Distilled water
Answer: c) Potassium hydroxide
7. Which of the following metals is commonly used as the anode in a lithium-ion battery?
a) Lithium
b) Nickel
c) Cobalt
d) Graphite
Answer: d) Graphite
8. Which type of battery is typically used as a backup power supply for computer systems?
a) Alkaline battery
b) Zinc-carbon battery
c) Nickel-cadmium battery
d) Lithium-ion battery
Answer: d) Lithium-ion battery
9. What is the primary disadvantage of lead-acid batteries?
a) High cost
b) Low energy density
c) Limited cycle life
d) Environmental concerns
Answer: c) Limited cycle life
10. What is the primary advantage of flow batteries compared to traditional batteries?
a) Higher energy density
b) Longer shelf life
c) Scalability for energy storage
d) Faster charging time
Answer: c) Scalability for energy storage
MCQ on lithium-ion battery | lead acid battery mcq
11. Which gas is produced at the anode during the electrolysis of brine (sodium chloride solution)?
a) Oxygen
b) Hydrogen
c) Chlorine
d) Carbon dioxide
Answer: c) Chlorine
12. What is the function of the separator in a battery?
a) Preventing short circuits
b) Regulating the voltage
c) Storing electrical energy
d) Catalyzing the electrochemical reaction
Answer: a) Preventing short circuits
13. Which type of battery has the highest energy density?
a) Alkaline battery
b) Nickel-metal hydride battery
c) Lithium-ion battery
d) Lead-acid battery
Answer: c) Lithium-ion battery
14. What is the approximate efficiency of water electrolysis for hydrogen production?
a) 50%
b) 75%
c) 90%
d) 100%
Answer: c) 90%
15. Which of the following is not a factor affecting the capacity of a battery?
a) Temperature
b) Voltage
c) Age
d) Current drain
Answer: b) Voltage
16. Which of the following is the primary electrolyte used in lead-acid batteries?
a) Sulfuric acid
b) Hydrochloric acid
c) Nitric acid
d) Acetic acid
Answer: a) Sulfuric acid
17. Which metal is commonly used as the cathode in electrolysis?
a) Copper
b) Zinc
c) Aluminum
d) Silver
Answer: a) Copper
18. What is the chemical symbol for the positive electrode in a battery?
a) P
b) N
c) C
d) A
Answer: c) C
19. Which type of battery is often used in remote controls and watches?
a) Nickel-cadmium battery
b) Alkaline battery
c) Lithium-ion battery
d) Zinc-carbon battery
Answer: d) Zinc-carbon battery
20. What is the primary advantage of nickel-metal hydride (NiMH) batteries compared to nickel-cadmium (NiCd) batteries?
a) Higher energy density
b) Longer lifespan
c) Lower cost
d) Faster charging time
Answer: b) Longer lifespan
lithium-ion battery mcq
21. What is the main function of an electrolyte in a battery?
a) To provide electrical conductivity
b) To store electrical energy
c) To regulate the voltage
d) To prevent corrosion
Answer: a) To provide electrical conductivity
22. Which gas is released at the positive electrode during the electrolysis of water?
a) Oxygen
b) Hydrogen
c) Nitrogen
d) Carbon dioxide
Answer: a) Oxygen
23. Which of the following is not a characteristic of a rechargeable battery?
a) Can be used multiple times
b) Converts chemical energy to electrical energy
c) Requires periodic recharging
d) Cannot be replaced or refilled
Answer: d) Cannot be replaced or refilled
24. What is the primary drawback of using hydrogen as an energy storage medium?
a) Low energy density
b) High cost
c) Limited availability
d) Environmental concerns
Answer: a) Low energy densit
25. Which type of battery chemistry is commonly used in hybrid electric vehicles (HEVs)?
a) Lithium-ion battery
b) Nickel-metal hydride battery
c) Lead-acid battery
d) Zinc-carbon battery
Answer: b) Nickel-metal hydride battery
26. Which of the following is not a primary component of a fuel cell?
a) Electrolyte
b) Cathode
c) Anode
d) Capacitor
Answer: d) Capacitor
27. What is the primary function of the electrolyte in a fuel cell?
a) To generate electrical energy
b) To store chemical energy
c) To facilitate ion conduction
d) To regulate the temperature
Answer: c) To facilitate ion conduction
28. Which type of battery chemistry is commonly used in small-scale electronics such as smartphones and laptops?
a) Lead-acid battery
b) Lithium-ion battery
c) Nickel-metal hydride battery
d) Zinc-carbon battery
Answer: b) Lithium-ion battery
29. Which gas is released during the discharge of a nickel-cadmium (NiCd) battery?
a) Hydrogen
b) Oxygen
c) Nitrogen
d) Cadmium
Answer: a) Hydrogen
30. What is the purpose of the separator in a fuel cell?
a) To prevent short circuits
b) To regulate the voltage
c) To store electrical energy
d) To catalyze the electrochemical reaction
Answer: a) To prevent short circuits
31. Which type of battery is commonly used in emergency lighting systems and alarm systems?
a) Alkaline battery
b) Nickel-cadmium battery
c) Lead-acid battery
d) Lithium-ion battery
Answer: c) Lead-acid battery
32. Which of the following is not a characteristic of a supercapacitor?
a) High energy density
b) Rapid charging and discharging
c) Long cycle life
d) Low specific power
Answer: d) Low specific power
33. Which gas is produced during the discharge of a lead-acid battery?
a) Hydrogen
b) Oxygen
c) Nitrogen
d) Sulfur dioxide
Answer: b) Oxygen
34. What is the primary advantage of using a flow battery for energy storage?
a) High energy density
b) Low cost
c) Long lifespan
d) Scalability
Answer: d) Scalability
35. Which of the following is an application of redox flow batteries?
a) Electric vehicles
b) Laptop computers
c) Grid-scale energy storage
d) Portable medical devices
Answer: c) Grid-scale energy storage
36. Which of the following is the most common anode material used in lithium-ion batteries?
a) Graphite
b) Copper
c) Aluminum
d) Nickel
Answer: a) Graphite
37. What is the primary function of the separator in a lithium-ion battery?
a) To prevent short circuits
b) To regulate the voltage
c) To store electrical energy
d) To catalyze the electrochemical reaction
Answer: a) To prevent short circuits
38. Which of the following is not a commonly used electrolyte in lithium-ion batteries?
a) Lithium carbonate
b) Lithium hexafluorophosphate
c) Lithium hydroxide
d) Lithium sulfur
Answer: d) Lithium sulfur
39. Which gas is produced during the discharge of a nickel-metal hydride (NiMH) battery?
a) Hydrogen
b) Oxygen
c) Nitrogen
d) Nickel
Answer: a) Hydrogen
40. What is the primary advantage of using a solid-state battery compared to traditional liquid electrolyte batteries?
a) Higher energy density
b) Longer lifespan
c) Safer operation
d) Faster charging time
Answer: c) Safer operation
Electrolysis and Storage of Batteries MCQs [Electricity Engineering]
41. Which of the following is not a component of a redox flow battery?
a) Electrolyte reservoirs
b) Membrane separator
c) Cathode and anode
d) Solid-state electrolyte
Answer: d) Solid-state electrolyte
42. What is the main purpose of a battery management system (BMS) in a rechargeable battery?
a) To control the charging and discharging process
b) To increase the energy density
c) To regulate the temperature
d) To convert electrical energy into chemical energy
Answer: a) To control the charging and discharging process
43. Which gas is produced during the discharge of a zinc-carbon battery?
a) Hydrogen
b) Oxygen
c) Nitrogen
d) Zinc
Answer: a) Hydrogen
44. What is the primary disadvantage of using lithium-ion batteries?
a) High cost
b) Low energy density
c) Limited lifespan
d) Environmental concerns
Answer: c) Limited lifespan
45. Which of the following is an example of a secondary battery?
a) Alkaline battery
b) Zinc-carbon battery
c) Lithium-ion battery
d) Fuel cell
Answer: c) Lithium-ion battery
46. What is the primary advantage of using a molten salt electrolyte in high-temperature electrolysis?
a) Higher efficiency
b) Lower cost
c) Safer operation
d) Longer lifespan
Answer: a) Higher efficiency
47. Which type of battery chemistry is commonly used in hearing aids and pacemakers?
a) Zinc-air battery
b) Lithium-ion battery
c) Nickel-cadmium battery
d) Alkaline battery
Answer: a) Zinc-air battery
48. Which gas is produced during the discharge of a fuel cell?
a) Hydrogen
b) Oxygen
c) Nitrogen
d) Carbon dioxide
Answer: b) Oxygen
49. What is the main advantage of using vanadium redox flow batteries for energy storage?
a) High energy density
b) Rapid charging and discharging
c) Long lifespan
d) Low cost
Answer: c) Long lifespan
50. Which metal is commonly used as the cathode in alkaline batteries?
a) Zinc
b) Nickel
c) Cadmium
d) Iron
Answer: b) Nickel
MCQ on Battery Charging System
51. What is the function of the electrolyte in a supercapacitor?
a) To generate electrical energy
b) To store chemical energy
c) To facilitate ion conduction
d) To regulate the temperature
Answer: c) To facilitate ion conduction
52. Which gas is produced during the electrolysis of brine using a membrane cell?
a) Hydrogen
b) Oxygen
c) Chlorine
d) Sodium
Answer: c) Chlorine
53. Which type of battery is commonly used in backup power systems for renewable energy installations?
a) Lithium-ion battery
b) Lead-acid battery
c) Nickel-metal hydride battery
d) Zinc-carbon battery
Answer: b) Lead-acid battery
54. What is the primary advantage of using a solid-state electrolyte in batteries?
a) Higher energy density
b) Longer lifespan
c) Safer operation
d) Faster charging time
Answer: c) Safer operation
55. Which gas is released during the discharge of a nickel-iron (NiFe) battery?
a) Hydrogen
b) Oxygen
c) Nitrogen
d) Iron
Answer: d) Iron
56. What is the primary disadvantage of using lithium-ion batteries in electric vehicles?
a) Limited availability of lithium
b) High cost of production
c) Long charging time
d) Low energy density
Answer: c) Long charging time
57. Which of the following is not a commonly used cathode material in lithium-ion batteries?
a) Lithium cobalt oxide
b) Lithium manganese oxide
c) Lithium nickel cobalt aluminum oxide
d) Lithium iron phosphate
Answer: c) Lithium nickel cobalt aluminum oxide
58. What is the function of the separator in a supercapacitor?
a) To prevent short circuits
b) To regulate the voltage
c) To store electrical energy
d) To catalyze the electrochemical reaction
Answer: a) To prevent short circuits
59. Which gas is produced during the discharge of a zinc-air battery?
a) Hydrogen
b) Oxygen
c) Nitrogen
d) Zinc
Answer: b) Oxygen
60. What is the main advantage of using sodium-ion batteries?
a) Lower cost compared to lithium-ion batteries
b) Higher energy density than lithium-ion batteries
c) Faster charging time than lithium-ion batteries
d) Longer lifespan than lithium-ion batteries
Answer: a) Lower cost compared to lithium-ion batteries
61. Which type of battery is commonly used in power tools and electric vehicles?
a) Lithium-ion battery
b) Nickel-metal hydride battery
c) Lead-acid battery
d) Zinc-carbon battery
Answer: a) Lithium-ion battery
62. Which gas is produced during the discharge of a hydrogen fuel cell?
a) Hydrogen
b) Oxygen
c) Nitrogen
d) Water vapor
Answer: b) Oxygen
63. What is the primary disadvantage of using solid-state batteries?
a) Limited energy density
b) High cost of production
c) Low stability and lifespan
d) Slow charging and discharging rates
Answer: b) High cost of production
64. Which type of battery is commonly used in remote sensing and monitoring applications due to its long shelf life?
a) Alkaline battery
b) Lithium-ion battery
c) Nickel-cadmium battery
d) Zinc-carbon battery
Answer: a) Alkaline battery
65. What is the main advantage of using lithium-sulfur batteries?
a) Higher energy density compared to lithium-ion batteries
b) Longer lifespan than lithium-ion batteries
c) Safer operation compared to lithium-ion batteries
d) Faster charging time than lithium-ion batteries
Answer: a) Higher energy density compared to lithium-ion batteries
66. Which metal is commonly used as the anode in a magnesium-ion battery?
a) Lithium
b) Sodium
c) Magnesium
d) Aluminum
Answer: c) Magnesium
67. What is the main advantage of using solid-state electrolytes in batteries?
a) Higher energy density
b) Safer operation
c) Lower cost
d) Faster charging time
Answer: b) Safer operation
68. Which gas is produced during the electrolysis of water using a membrane cell?
a) Hydrogen
b) Oxygen
c) Chlorine
d) Sodium
Answer: a) Hydrogen
69. Which type of battery chemistry is commonly used in electric vehicles (EVs) due to its high energy density?
a) Lithium-ion battery
b) Nickel-metal hydride battery
c) Lead-acid battery
d) Zinc-carbon battery
Answer: a) Lithium-ion battery
70. What is the primary advantage of using a solid oxide electrolysis cell (SOEC) for hydrogen production?
a) High efficiency
b) Low cost
c) Compact size
d) Rapid hydrogen production
Answer: a) High efficiency
71. Which gas is produced at the cathode during the electrolysis of water?
a) Hydrogen
b) Oxygen
c) Chlorine
d) Sodium
Answer: b) Oxygen
72. Which type of battery is commonly used in renewable energy systems and off-grid applications?
a) Lithium-ion battery
b) Lead-acid battery
c) Nickel-metal hydride battery
d) Zinc-carbon battery
Answer: b) Lead-acid battery
73. What is the primary advantage of using a flow battery for energy storage compared to traditional batteries?
a) Higher energy density
b) Longer lifespan
c) Scalability
d) Faster charging time
Answer: c) Scalability
74. Which gas is produced during the discharge of a nickel-hydrogen (NiH2) battery?
a) Hydrogen
b) Oxygen
c) Nitrogen
d) Nickel
Answer: a) Hydrogen
75. What is the main advantage of using lithium-polymer batteries?
a) Flexibility in shape and size
b) Higher energy density compared to lithium-ion batteries
c) Lower cost compared to lithium-ion batteries
d) Faster charging time than lithium-ion batteries
Answer: a) Flexibility in shape and size
76. Which of the following is a common application of electroplating?
a) Water purification
b) Hydrogen production
c) Metal corrosion protection
d) Battery charging
Answer: c) Metal corrosion protection
77. Which metal is commonly used as the anode in a zinc-carbon battery?
a) Zinc
b) Carbon
c) Aluminum
d) Copper
Answer: a) Zinc
78. What is the main disadvantage of using nickel-cadmium (NiCd) batteries?
a) Limited lifespan
b) High cost
c) Low energy density
d) Environmental concerns
Answer: d) Environmental concerns